Clariant offers, via its Group Product Stewardship organization, professional service for customers and all Business Units. With these activities Clariant contributes to a high safety of products and prevents the business and customers from reputational or legal damages. The responsibility for the protection of consumers and the environment in the use of the products is given the highest priority. Thus Product Stewardship gives added value to the business and sustainability.
In the course of product development and product design, properties are evaluated with regard to the safe and environmentally compatible use of a material. Before a product is produced and marketed by Clariant, it undergoes a series of screenings according to and going beyond legal requirements. This is done to make sure that the product can be used without causing any harm to people and the environment during its entire life-cycle. Anyhow, the expertise in the Product Stewardship area ensures that Clariant products comply all relevant national and international chemicals legislation.
Safety through Assessment
Since 2007, the European Union (EU) has required that all chemical substances manufactured inside the EU and imported into the EU are being assessed in regards to the impact of these substances to humans and the environment (dossier). The EU authority ECHA (European Chemicals Agency) in Helsinki coordinates the recording, assessment and continued approval of all chemical substances on the basis of the dossiers (presented test results). To do this, it requests verifiable and authoritative statements from the chemical industry. The research methods must be perfectly reproducible and is normally associated with considerable effort and expense. Clariant is committed to fully completing these requirements on time and thereby strives for the highest levels of cost-efficiency while undertaking as little animal-testing as possible, as permitted by regulation.
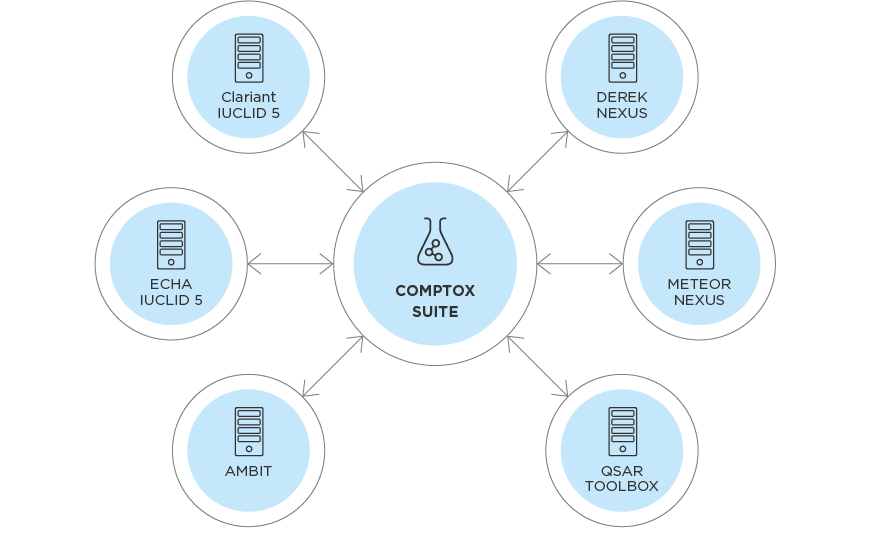
The CompTox Suite – assessment of chemicals without additional animal studies
Chemicals must be registered to protect humans and the environment. A registration requires substance data, proof of safe use of the substance and submission of substance data and assessments to the competent authorities.
»The CompTox Suite marks a milestone in the assessment of chemical substances.«
DATA ACQUISITION WITH THE COMPTOX SUITE
- Forecasts of chemical safety profiles, product composition and improvements
- Targeted development by means of excluding harmful structures
- No (animal) testing
- Use of existing data on similar chemical structures
- Filling of data voids based on read-across and QSAR1 methods
- Support for category approach (registration of element groups)
- No (animal) testing
- Avoidance of animal testing
- Reduction of research and testing costs
- Faster research and development of new products
- Improved performance of laboratories and businesses
- Standardized reporting format
1 QSAR: Quantitative Structure-Activity Relationship
REACH (Registration, Evaluation, Authorization and Restriction of Chemicals), the chemicals law of the European Union, is one of the most stringent sets of regulations for the authorization of substances worldwide. With data generated for a REACH registration, the requirements of other countries can essentially also be fulfilled with only a few exceptions. The required toxicological data are mainly determined by extensive animal testing which are time and cost intensive.
For registration, the legal demand, as well as Clariant’s own animal welfare policy of minimizing animal testing must be followed. This can be done by using existing data on the substance itself or on similar substances.
The toxicity profile of a substance is determined by its chemical structure. Computational toxicology is a process that aims to predict toxic properties of chemical substances based on available data and thereby avoid animal experiments.
Clariant has been working for years to replace the required animal testing with alternative methods. The innovative approach taken by Clariant with its CompTox Suite comprises several elements, the first is that it combines and integrates the functionalities of the computational methods of AMBIT with high quality (eco)toxicity data from the REACH database IUCLID.
AMBIT is an open source software with attached database for data management and investigation of chemical structures and partial structures, developed by LRI1 and CEFIC2. It helps with the development of substances giving the possibility of modelling and simulation.
The AMBIT database contains more than 450 000 chemical structures and their identifiers (CAS, EINECS, InChI) as well as information about molecular descriptions, investigational data, literature references and REACH relevant PBT descriptions.
The software can also communicate with other programs and access data of different external quality assured and REACH relevant databases. These data are assigned to certain chemical structures or partial structures and stored accordingly. Frequently repeated actions can be automated.
1 LRI (Long Range Research Initiative) is a research program to assure the safety and harmlessness of chemical products for humans and the environment, initiated by CEFIC2 and ICCA3.
2 CEFIC is the European Chemical Industry Council. It represents 29 000 companies in Europe with 1.2 million employees.
3 ICCA is the International Council of Chemical Associations.
In addition, the CompTox Suite links also computational toxicology prediction tools with Ambit. This link enables the prediction of toxicity from chemical structures, structural moieties, metabolites and allows read-across and category approaches as useful techniques in the safety (hazard and/or risk) assessment of chemicals. In the last step of the CompTox Suite experimental verification is possible using analytical methods to detect predicted metabolites in urine or blood samples – if required. As a result, the number of animal experiments, especially long-term studies to be carried out for the assessment of chemicals, can be significantly reduced.
Proactive product safety assessment of innovation projects by CompTox contributes to innovation strategy, sustainable products and competition advantage: health risks and other hazardous properties of substances in new developments can be recognized or even additional assessments of already existing compounds can be recognized and taken into account more easily from already available data.
Major contribution to improving animal protection: The CompTox Suite uses existing toxicological data of similar substances and modern calculation methods to fill data gaps for registrations and thereby minimizes animal experiments.
Clariant’s seven Business Units include: Additives; Catalysts; Functional Minerals; Industrial & Consumer Specialties; Oil & Mining Services; Pigments. VIEW ENTIRE GLOSSARY
REACH is an E.U. regulatory framework for the registration, evaluation and authorization of chemicals. VIEW ENTIRE GLOSSARY
REACH is an E.U. regulatory framework for the registration, evaluation and authorization of chemicals. VIEW ENTIRE GLOSSARY
